
[ad_1]
CMS launched a steerage on June 30, 2023 offering further element on on how the utmost truthful charge (MFP) can be calculated. The record additionally has just about 100 pages of public enter feedback. A abstract of a few key issues are beneath. Of maximum hobby is that CMS is taking a ‘reference charge’ based totally solution to atmosphere MFP.
- Surroundings MFP with regard pricing. “CMS will use the Phase D web charge(s) (‘web charge(s)’) and/or ASP(s) [average sales price] of the healing choice(s).” CMS says it’s going to then imagine changes in accordance with different elements, however it sounds as if that reference pricing is the principle manner for atmosphere MFP. CMS stated it’s going to “take a qualitative manner” to adjusting the beginning negotiation charge in accordance with the original traits of the drug and its healing possible choices. Moreover, notice that CMS will imagine the costs of generic and biosimilar merchandise within the package of healing possible choices. If there are not any healing possible choices, CMS will imagine a beginning negotiating charge in accordance with the FSS or “Large 4 Company”66 charge (“Large 4 charge”).
- Little need of QALYs. CMS explicitly said that it will no longer use quality-adjusted existence years (QALYs) as a part of MFP. What is going to they imagine? Results akin to treatment, survival, progression-free survival, advanced morbidity, advanced signs or affected person reported results may well be thought to be.
- Productiveness affects. CMS stated that it’s going to come with productiveness affects for sufferers, however isn’t making an allowance for productiveness affects of a remedy on caregivers.
- Caregiver point of view. CMS stated that they “…may additionally imagine the caregiver point of view to the level that it displays without delay upon the revel in or related results of the affected person taking the chosen drug.” It isn’t transparent how caregiver burden can be taken into consideration if most effective that is related to the affected person taking the drug.
- Availability of generic medication. When generic or biosimilars are to be had, MFP is probably not related. CMS said that they are going to use knowledge from Prescription Drug Match (PDE) knowledge and Reasonable Producer Value (AMP) to tell this discision.
- Orphan drug designation made up our minds by way of FDA, no longer CMS. CMS is not going to imagine withdrawn orphan designations or withdrawn approvals as disqualifying a drug from the Orphan Drug Exclusion from MFP negotiation.
- Confidentiality of knowledge all the way through negotiation. CMS is not going to publicly speak about ongoing negotiations previous to the discharge of the reason of the utmost truthful charge (MFP) until a Number one Producer publicly discloses data in regards to the negotiation procedure.
- Public rationalization of MFP. CMS will put up an evidence of the way MFP was once derived sooner than March 1 every 12 months previous to MFP going into impact.
- Use of scientific effectiveness and price effectiveness to decide MFP. CMS said “CMS reaffirmed that it’s going to no longer [emphasis mine]use proof from comparative scientific effectiveness analysis in a fashion that treats extending the lifetime of a person who’s aged, disabled, or terminally unwell as of decrease worth than extending the lifetime of a person who’s more youthful, nondisabled, or no longer terminally unwell. CMS additionally clarified that, for preliminary charge applicability 12 months 2026, it’s going to evaluate cost-effectiveness measures and research that use such measures to decide whether or not the measure used is also thought to be in keeping with phase 1194(e)(2) of the Act. On the other hand, whilst such measures is also thought to be, they are going to no longer [emphasis mine] be used to regulate the preliminary be offering if the measure does no longer supply related data or isn’t authorized in keeping with phase 1194(e)(2) of the Act and phase 1182(e) of the Act.”
- Unmet clinical want. CMS will imagine a drug to have unmet clinical want if there are “no different remedy choices exist or current remedies don’t adequately deal with the illness or situation.” This decision can be evaluated one after the other for every indication. CMS’s manner can be knowledgeable by way of FDA steerage.
- Producer-specific knowledge. CMS changed the in depth knowledge producers are anticipated to post.
The information issues that CMS will imagine for making changes to MFP past reference pricing will come with:
- Producer R&D prices. If a Number one Producer has no longer recouped its R&D prices, CMS might 151 imagine adjusting the initial charge upward. Conversely, if a Number one Producer has recouped its R&D prices, CMS might imagine adjusting the initial charge downward or follow no adjustment
- Present unit prices of manufacturing and distribution of the drug. CMS might imagine adjusting the initial charge downward if the unit prices of manufacturing and distribution are not up to the initial charge, or upward if the unit prices of manufacturing and distribution are more than the initial charge
- Prior Federal monetary enhance for novel healing discovery and construction with appreciate to the drug. CMS might imagine adjusting the initial charge downward if investment for the invention and construction of the drug was once gained from Federal resources. It isn’t transparent how this is able to function since maximum drug–no less than within the elementary science segment–gained some enhance from Federal resources even supposing not directly.
- Knowledge on pending and licensed patent programs or exclusivities identified by way of the FDA, and programs and approvals underneath phase 505(c) of the FD&C Act or phase 351(a) of the PHS Act for the drug. If there are not any long run competitor medication coming to marketplace, that might affect CMS designation that the drug will proceed to fulfill an unmet clinical want.
- Marketplace knowledge and income and gross sales quantity knowledge for the drug in america. If the typical industrial web charge is not up to the initial charge, CMS might imagine adjusting the initial charge downward. If the typical industrial web charge is larger than the initial charge, CMS might imagine adjusting the initial charge upward.
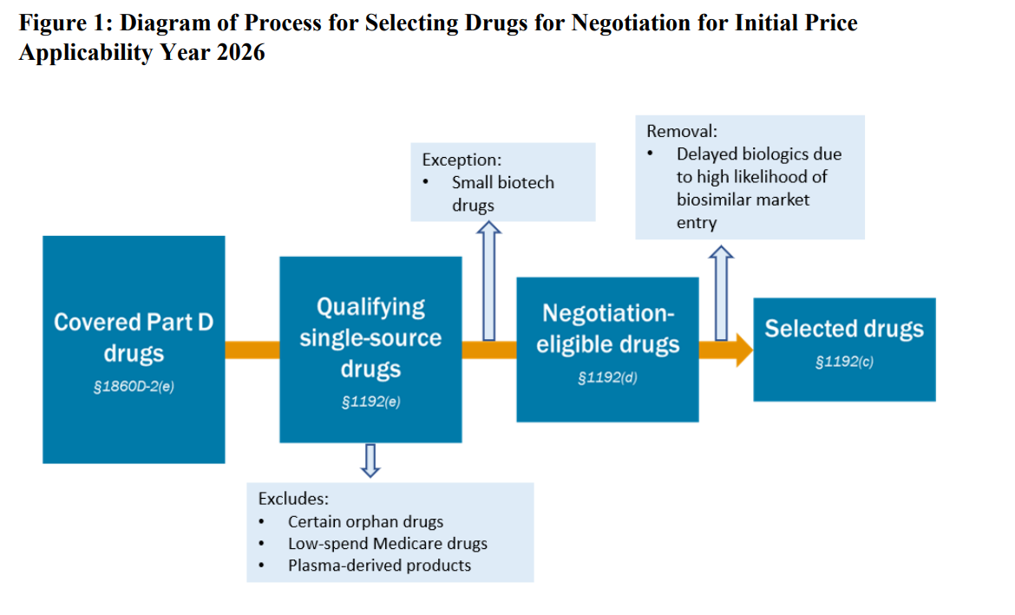
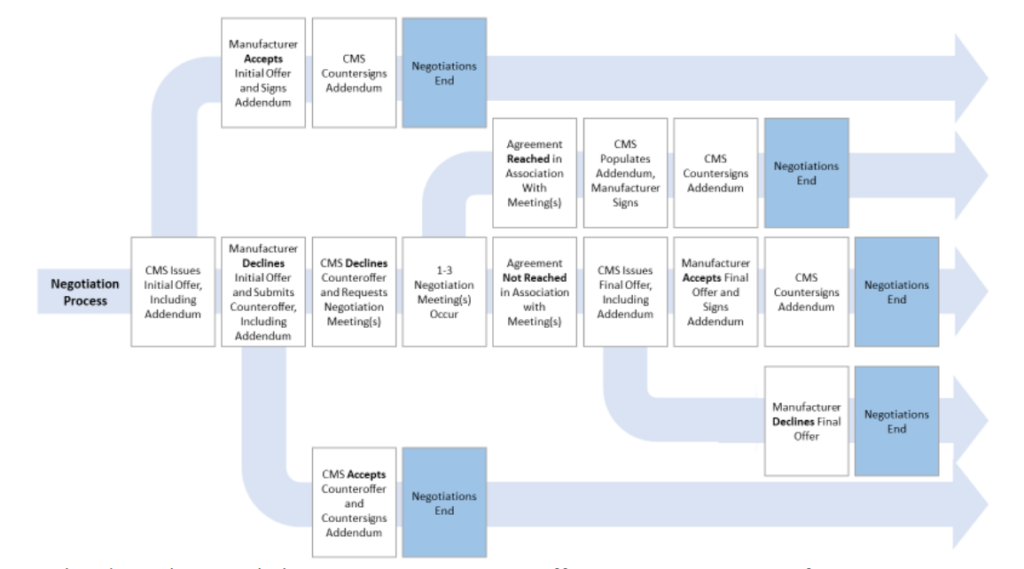
The whole CMS steerage is to be had right here.
[ad_2]